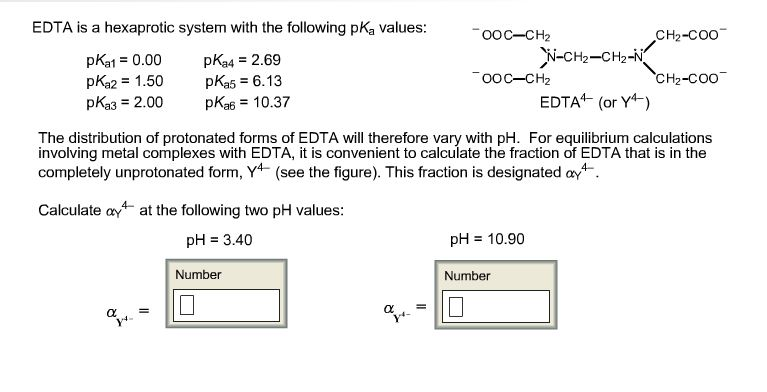
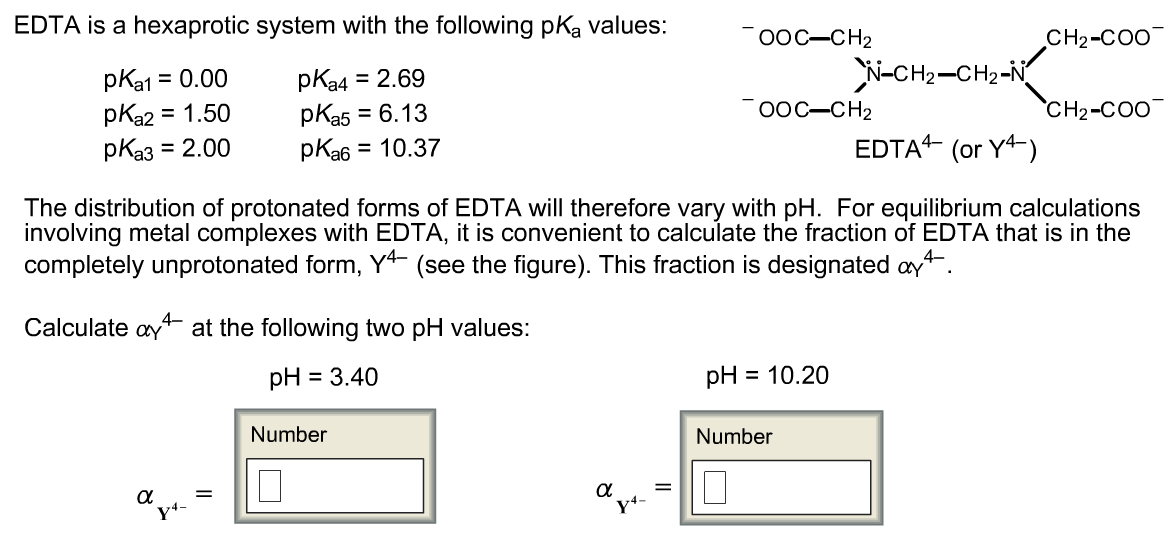
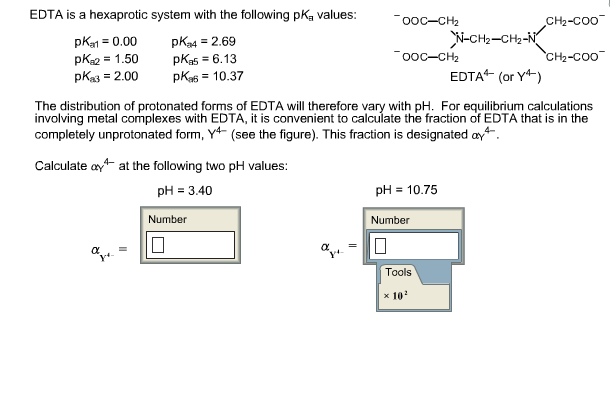
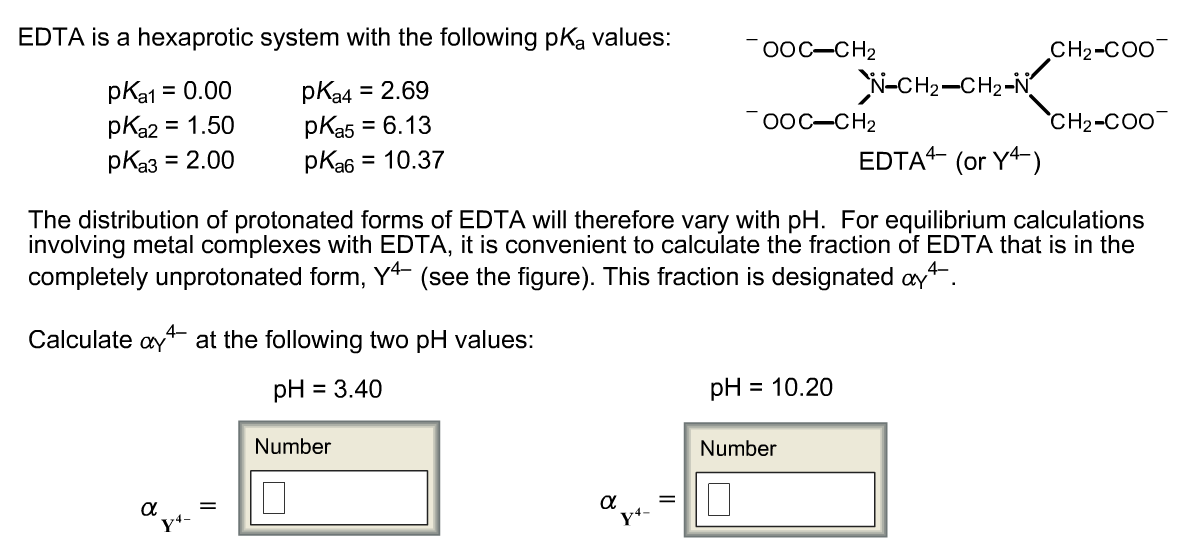
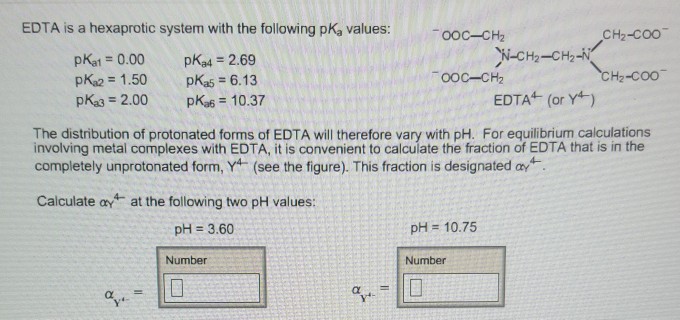
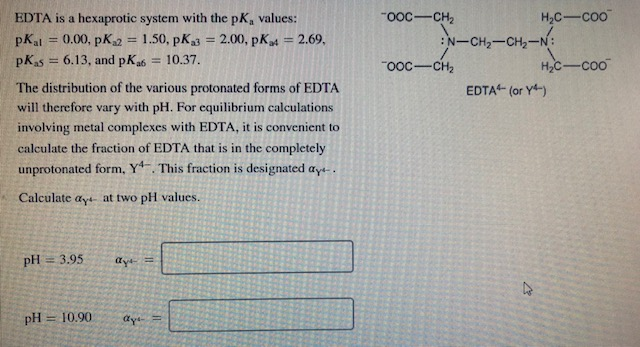
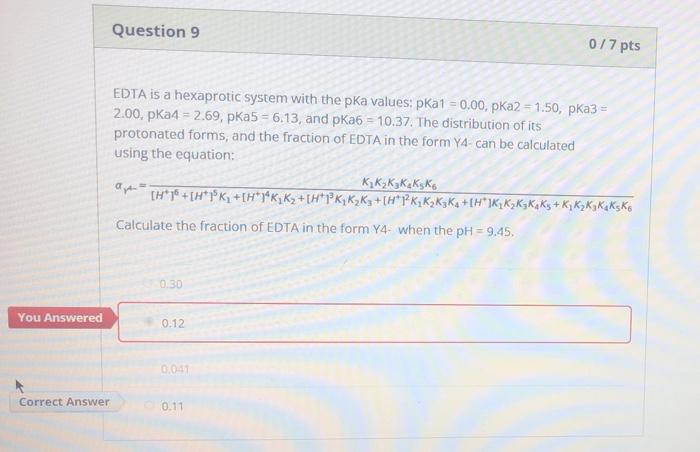
Edta Is A Hexaprotic System With The Following Pka Values
Edta is a hexaprotic system with the following pka values. EDTA Formation Constants EDTA is a hexaprotic weak acid that complexes 11 with metal cations-Notice that the first 4 protons are much more acidic than the last two so the dominant form of EDTA in solution will be H2Y2-. Actually the concentration off let two positive is not zero. The distribution of the various protonated forms of edta will therefore vary with ph.
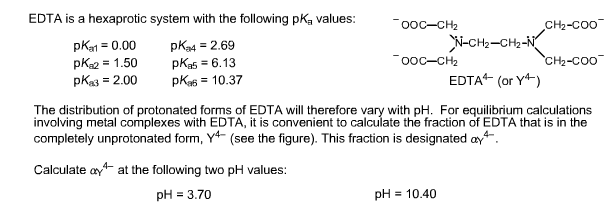
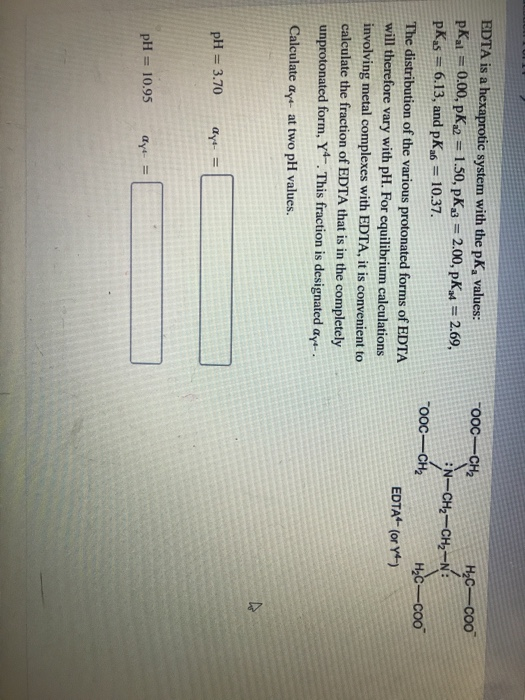
EDTA is a hexaprotic system with the pKa values. Show transcribed image text EDTA is a hex aprotic system with the following pKa values. By direct titration or through an indirect sequence of reactions virtually every element of the periodic table can be measured with EDTA.
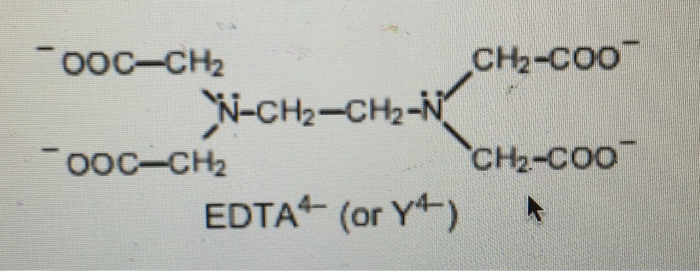
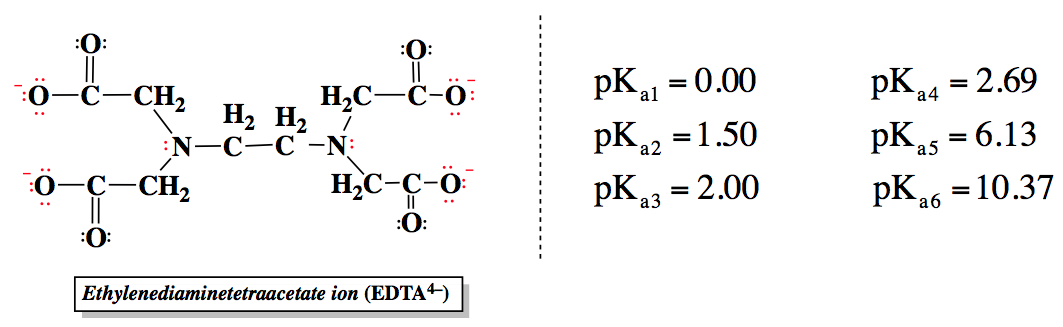
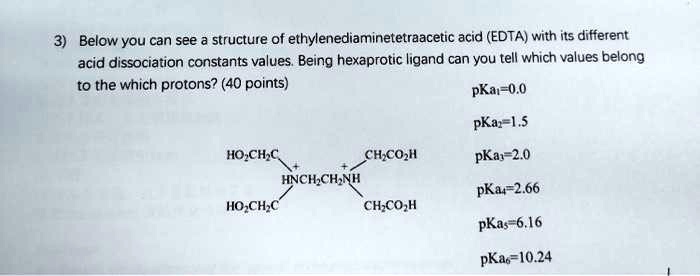
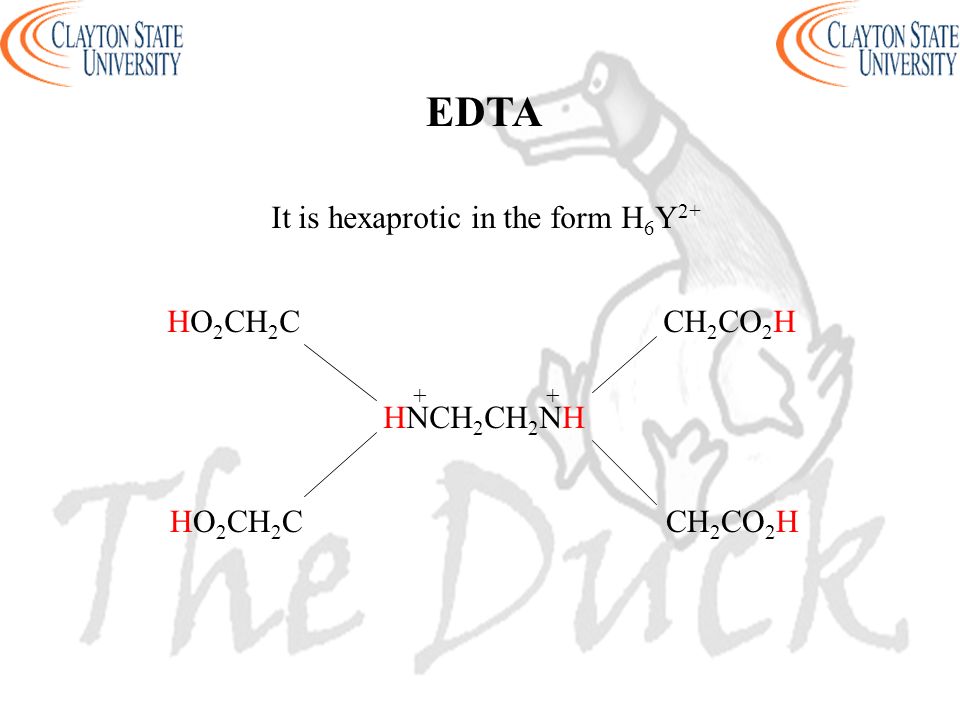
Ad Product selection guide for all protein analysis needs. EDTA is a hexaprotic system with the following pKa values. 0oc-CH H2C-COO pKal 000 pK2 150 pKs 200 pKa4 269 N-CH2-CH2-N.
The first four pK values apply to carboxyl. The distribution of protonated forms of. Ad Product selection guide for all protein analysis needs.
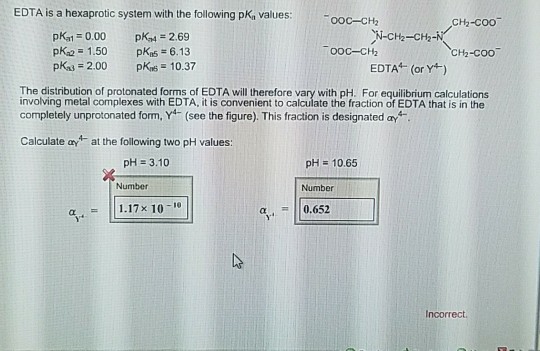
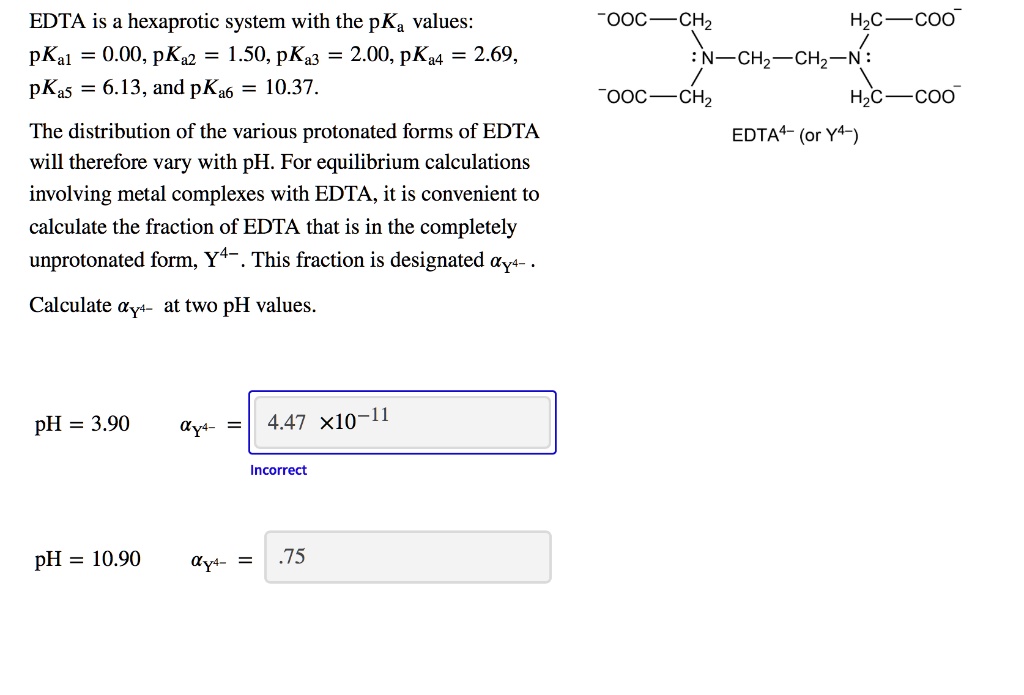
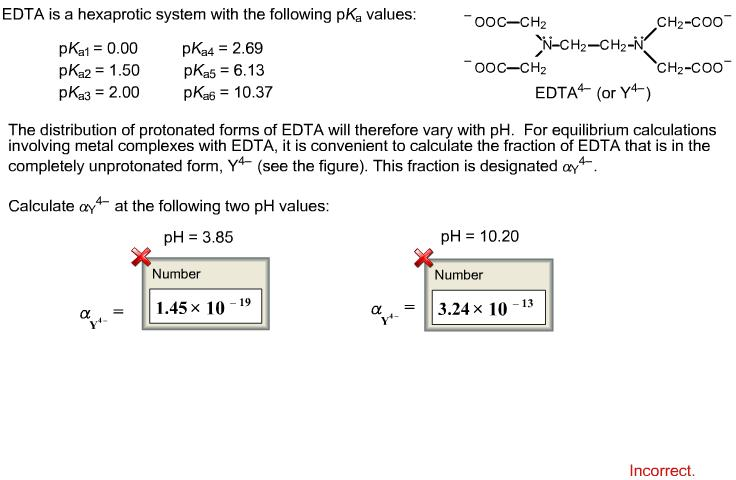
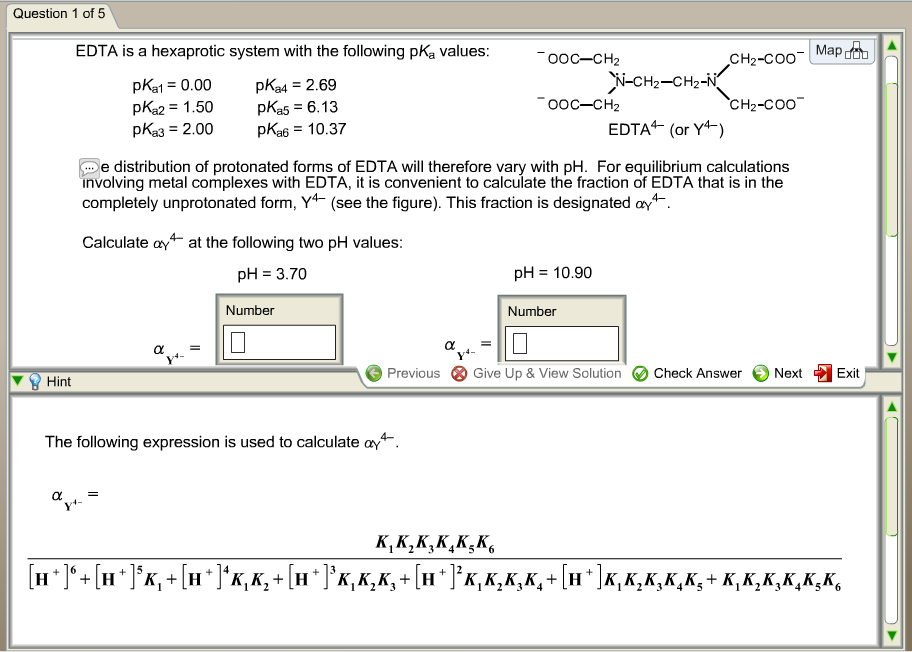
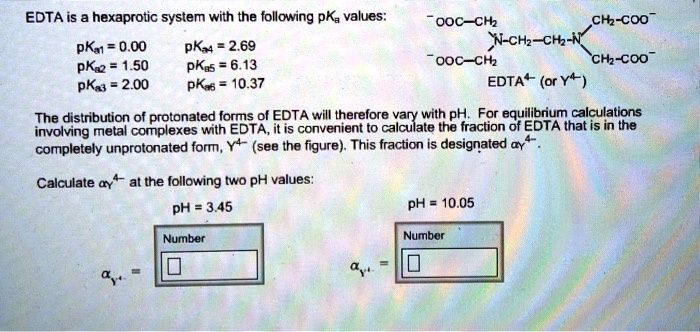
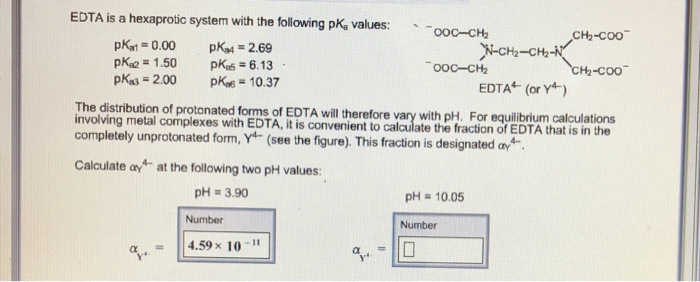
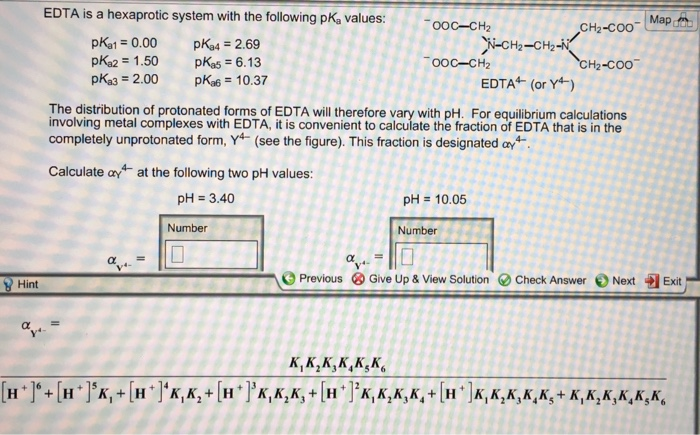
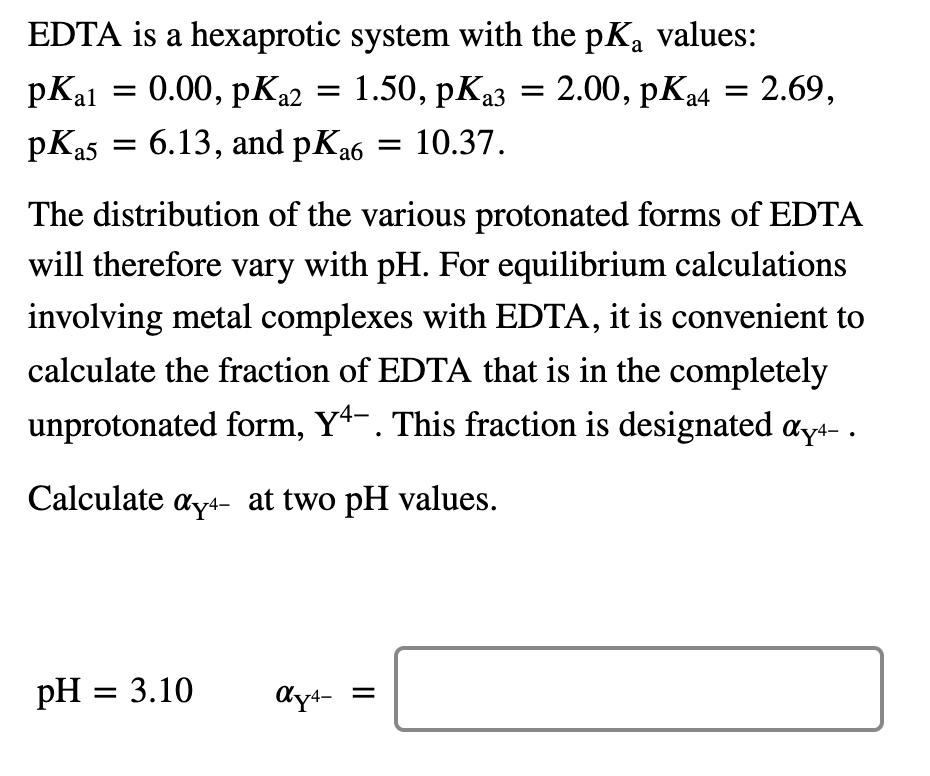
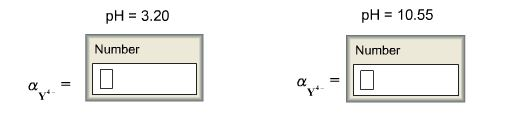
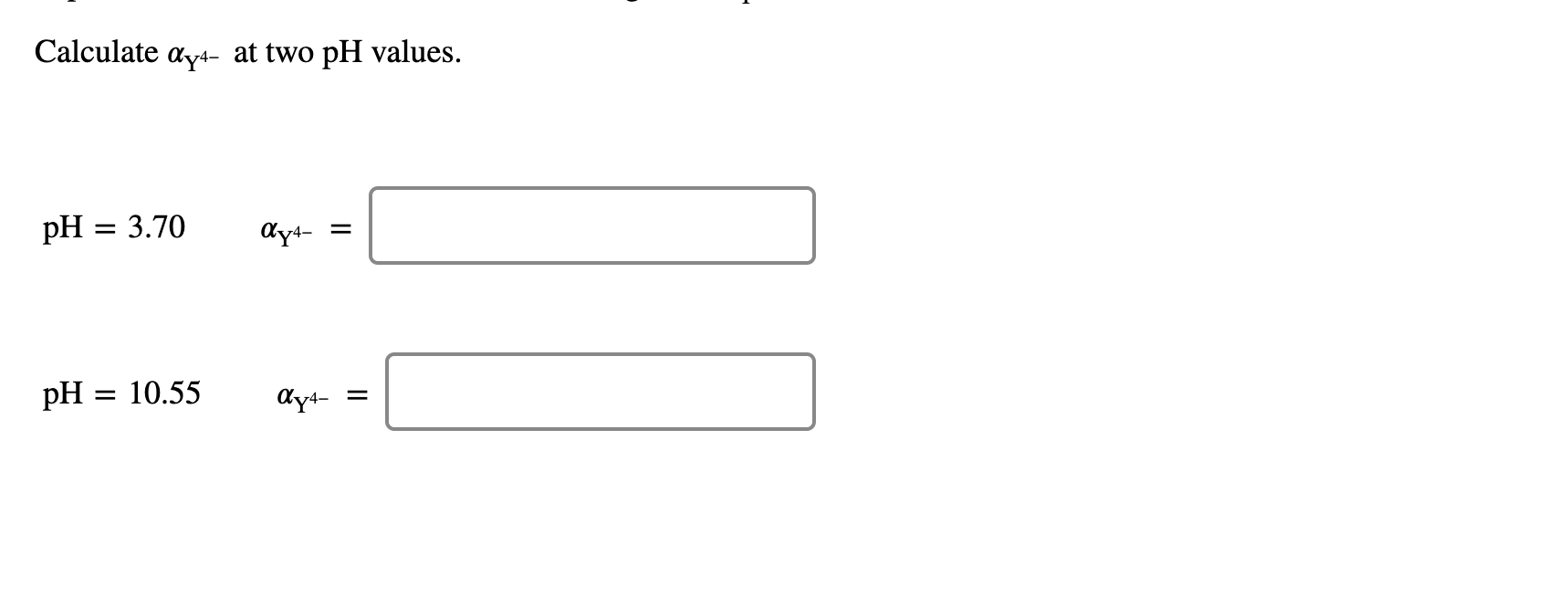
EDTA is a hexaprotic system with the following pKa values. PKa1 000 pKa4 269 pKa2150 pKa5 613 pKa3 200 pKa6 1037 The distribution of protonated forms of EDTA will therefore vary with pH. EDTA is a hexaprotic system with the following pKa values.
Edta is a hexaprotic system with the pka values. Mn H 2Y 2- MYn-4 2H By Le Chateliers Principle the complex will dissociate at low pHs and it will be more. PKa1000 pKa2150 pKa3200 pKa4269 pKa5613 and pKa61037.
We substitute the values off after the reaction in tow. EDTA is a hexaprotic system with the pKa values.
Learning centers handbooks protocols scientific posters citations get support.
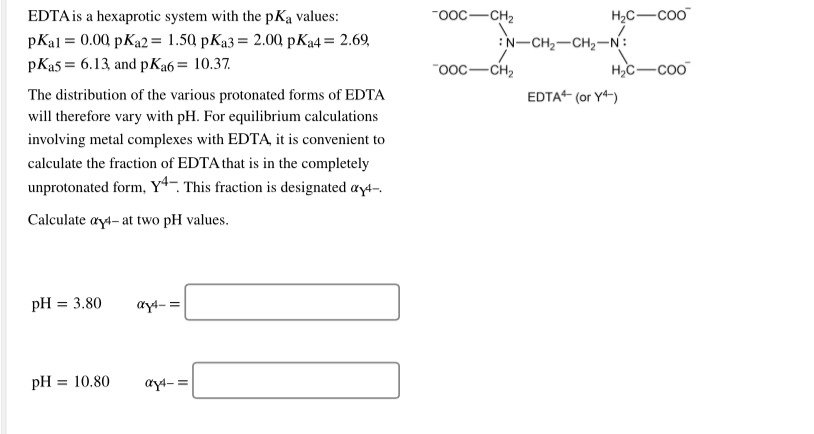
EDTA is a hexaprotic system designated H6Y2. Get 20 off grade yearly subscription. Extraction clean up detection. 00C-CH2 HC-coO The distribution of the various protonated forms of EDTA EDTA- or Y4- will therefore vary with pH. Ad Product selection guide for all protein analysis needs. EDTA is a hexaprotic system with the following pKa values. EDTA Formation Constants EDTA is a hexaprotic weak acid that complexes 11 with metal cations-Notice that the first 4 protons are much more acidic than the last two so the dominant form of EDTA in solution will be H2Y2-. EDTA is by far the most widely used chelator in analytical chemistry. EDTA is a hexaprotic system with the following pKa values.
The distribution of protonated forms of. Ad Product selection guide for all protein analysis needs. PKa1 000 pKa4 269 pKa2 150 pKa5 613 pKa3 200 pKa6 1037 The distribution of protonated forms of EDTA will therefore vary with pH. Learning centers handbooks protocols scientific posters citations get support. We substitute the values off after the reaction in tow. Cas 2H2Og S. Show transcribed image text EDTA is a hex aprotic system with the following pKa values.






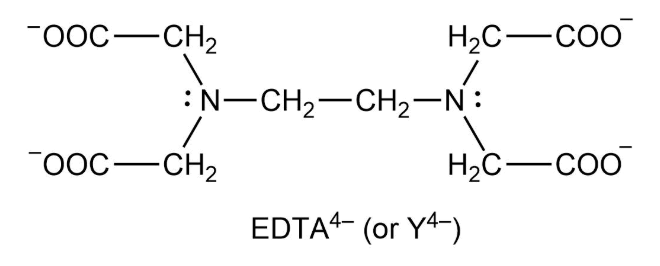











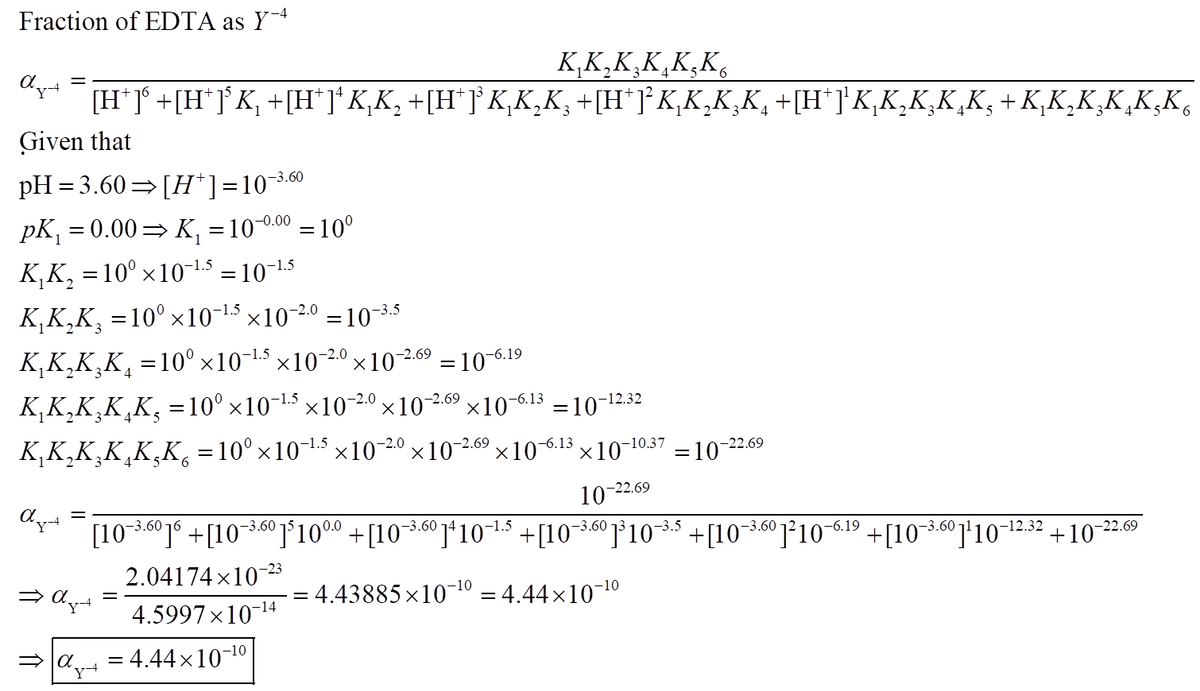

Post a Comment for "Edta Is A Hexaprotic System With The Following Pka Values"